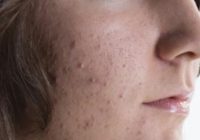
Amzeeq Now Available for Moderate to Severe Acne – Monthly Prescribing Reference
Amzeeq (minocycline; Foamix) topical foam 4% will soon be available (January 13, 2020) by prescription for the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients aged 9 years and older. Amzeeq utilizes Foamix’s proprietary Molecule Stabilizing Technology (MST) platform to deliver minocycline, a tetracycline-class drug, in a foam formulation; it… Read More »